Our Research
Natural Products for Drug and Supplement Development
The fields of medicinal chemistry, synthetic organic chemistry, molecular modeling, natural product chemistry, and fluorine chemistry are combined in our laboratory for creating innovative approaches for drug discovery and dietary supplement development. Our group develops novel therapeutics for diseases that have no or few existing treatment options, including neurodegenerative disorders, drug addiction, and cancer. Each research project starts with natural products as the initial inspiration for drug discovery.
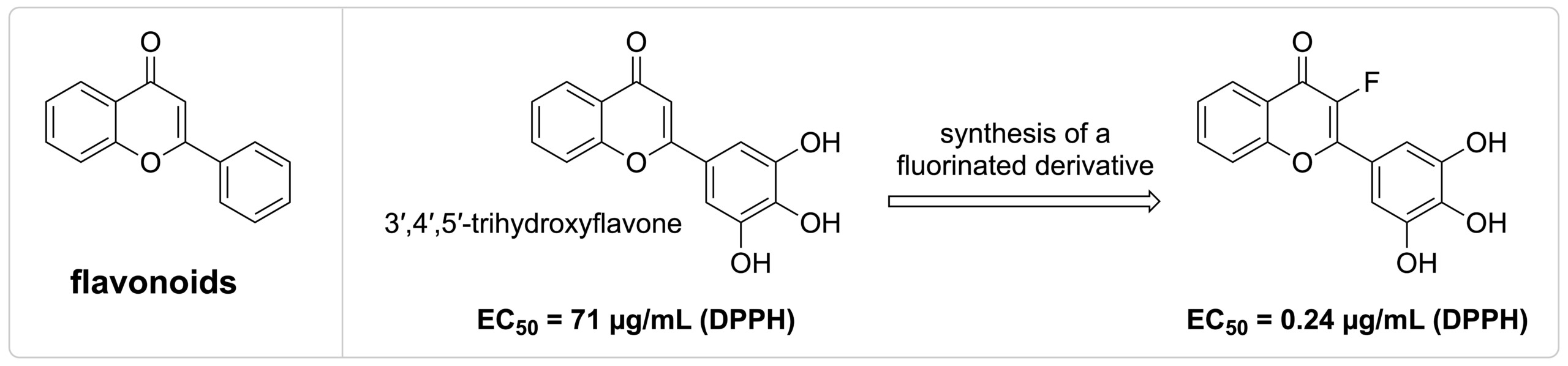
Currently, our research is focused on flavonoids, in which we directly modify their structures, develop strategies for the total synthesis of these novel targets, and explore their biological activities. We have designed and synthesized fluorinated derivatives of flavones, which show enhanced antioxidant activity. Additionally, quantitative 19F NMR is a method that is frequently utilized in our projects for monitoring antioxidant activities, prodrug conversion, and reaction progression.
Synthetic Methodology
Our other area of current research is developing synthetic methods for the modification of organic molecules and to install fluorine atoms. The cleavage of carbon-carbon bonds is also investigated in our research group to remodel the structure of complex compounds. The field of synthetic organic chemistry and fluorine chemistry are blended in our laboratory for the synthesis of biologically important molecules.
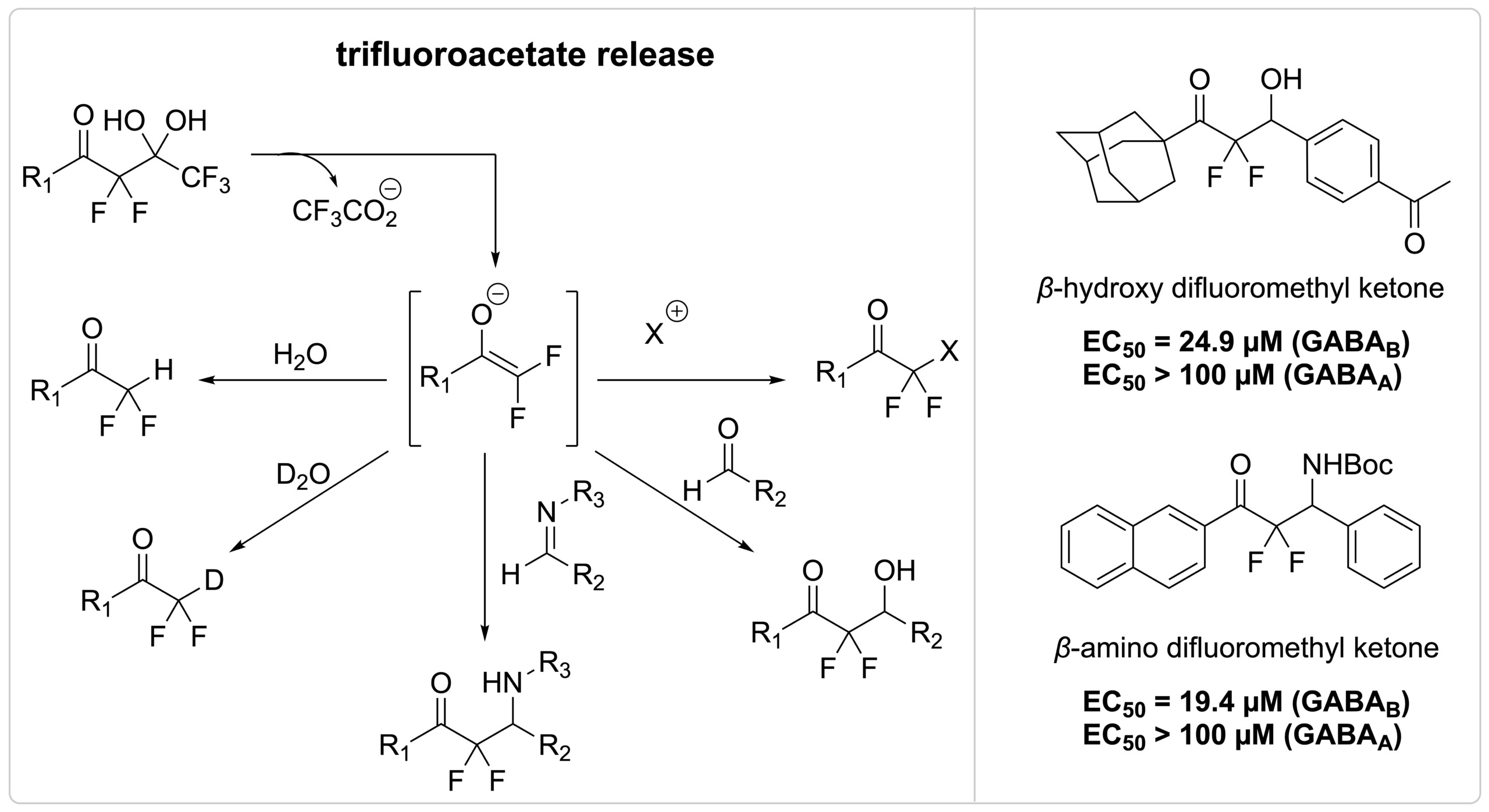
We have discovered that the release of trifluoroacetate is a powerful approach to break carbon–carbon bonds and generate difluoroenolates and other fluorinated intermediates. Our method has opened new avenues in synthetic chemistry for the creation of fluorinated organic molecules and includes reactions such as halogenation, deuteration, the aldol reaction, and imine-addition reactions. We employed this strategy to develop a novel class of selective agonist for the GABAB receptor, the β-hydroxy difluoromethyl ketones and β-amino difluoromethyl ketones.
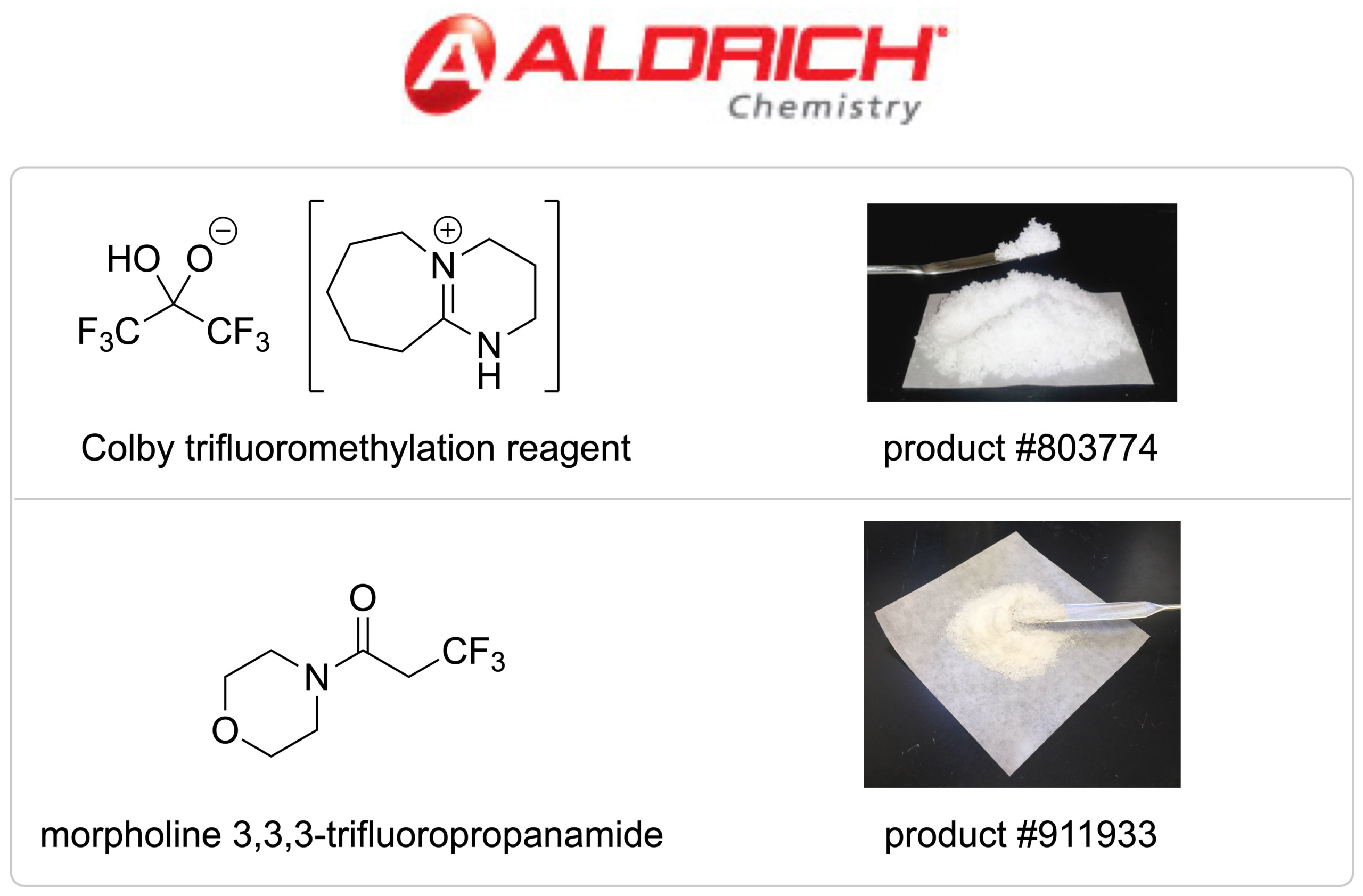
Reagents
Our research group is also interested in translating discoveries from the laboratory to the private sector and partnering with industry. We have two reagents that are commercialized with MilliporeSigma through Aldrich Chemistry. The first reagent is the Colby trifluoromethylation reagent, which we invented in 2013. The second is morpholine 3,3,3-trifluoropropanamide which can be used to construct (E)-β-fluoro-α,β-unsaturated amides, developed in 2020.